Dedicated Pharma Systems
On-Shelf Solutions, Ready to Implement
Where Things Really Get Done
Vial Handling Systems
Including LYO Loading/Unloading Solutions
Data Integrity Systems
Vial /Ampoule / Solid / Simi-Solid and Syrup Production Lines
Machine Vision Systems
Smart Inspection Camera Solutions with Detection & Rejection Solutions
Incubation Systems
Upgradation and Compliance with Accurate and Precise Readings
Modern SCADA Systems
Smart, Modular, Integral, and Complied Solutions
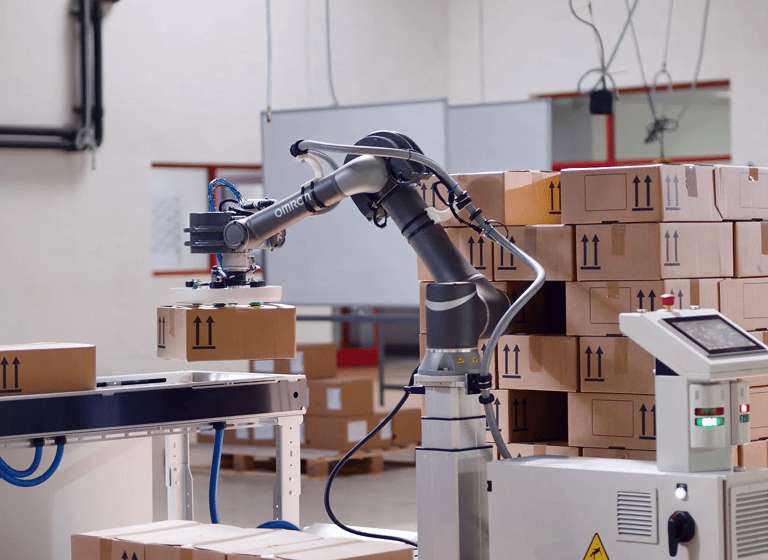
Robotic Packaging Systems
Including Carton Erectors, Handeling, and Conveying Solutions
Robotic Palletizing Systems
Including Pallet Dispensers and Assorting Solutions
Vial Handling Systems
Our vial handling solutions are custom-designed and dedicated to automating vial orientation, sorting, and transfer across sterile processes using Siemens or Beckhoff Automation and motion platforms. Features include servo conveyors, rotating tables, and synchronization with upstream/downstream equipment.
Cleanroom-ready and high-speed with GMP compatibility
Handles bulk and nested formats
Includes accumulation buffers and smart rejection modules
SCADA control and compliance with FDA 21 CFR Part 11 Guidelines
Special handling modules for Loading/Unloading Manual/Automatic LYO systems
Reference Project
Automated Vial Handling Line in Sterile Area
Design, supply, installation, programming, testing, commissioning, and validation of a complete automated vial handling system (loading/unloading to/from lyophilized machine up to sterile crimping). Fully compliant with FDA 21 CFR Part 11 & GMP standards. Hikma Pharmaceuticals - 2022








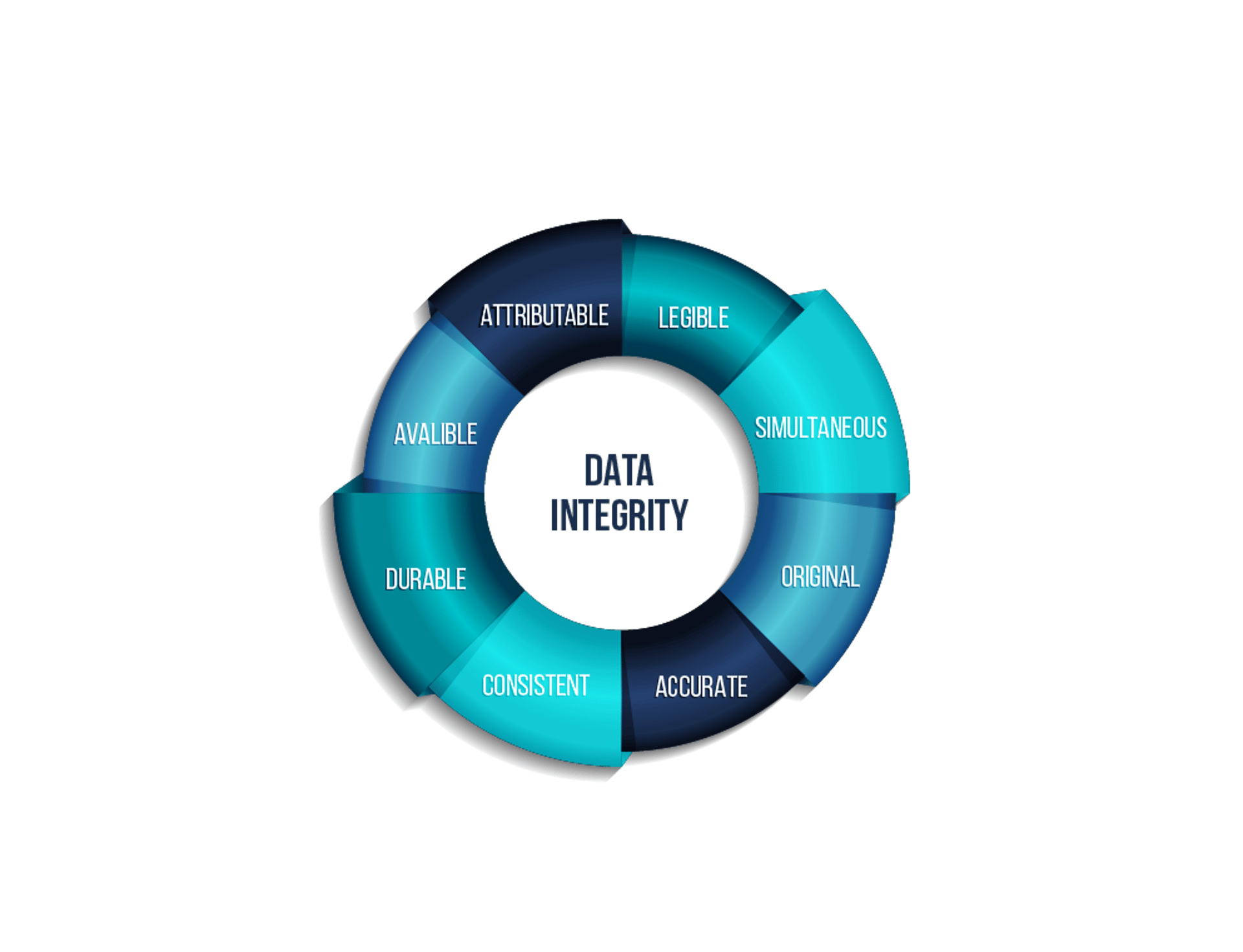
Data Integrity Solutions
We provide complete refurbishment services for vial and ampoule production lines, extending equipment lifetime while enhancing efficiency, compliance, and reliability. Our solutions ensure adherence to GMP standards, reduce downtime, and deliver cost-effective modernization. Additionally, we implement advanced Data Integrity solutions to secure electronic records and ensure full compliance with 21 CFR Part 11 and global regulatory requirements.
Reference Project
Upgrade of Oncology Vial/Ampoule Filling & Packaging Production Lines
Refurbishment of two ROTA production lines (washing, filling, crimping, labeling). Upgraded and validated control systems as a part of the client data integrity project. Hikma Pharmaceuticals - 2021
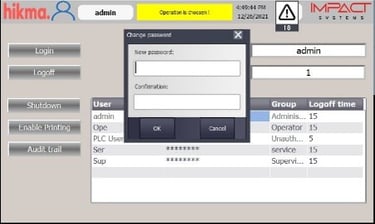
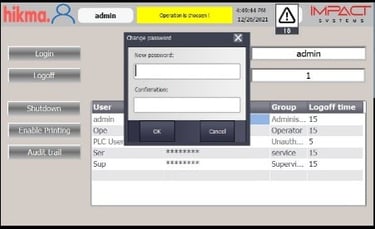
Automated Vial / Ampoule Lines Modernization
Integration of advanced, validated control systems aligned with Data Integrity requirements.
Implementation of secure electronic records and audit trails, ensuring full compliance with FDA 21 CFR Part 11 and GMP standards.
Comprehensive qualification and validation (IQ/OQ/PQ) to guarantee regulatory approval and operational reliability.
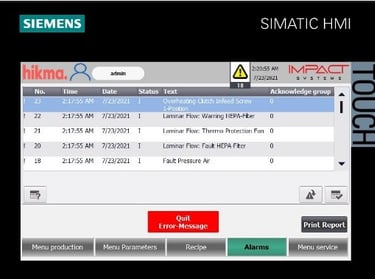
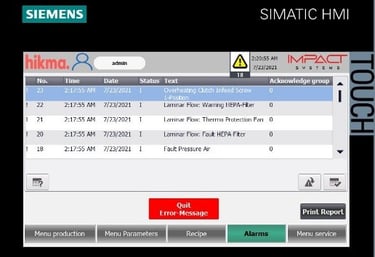
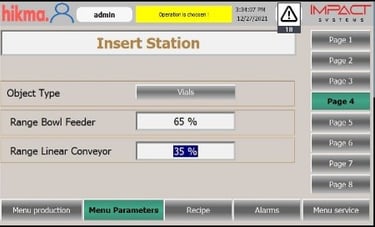
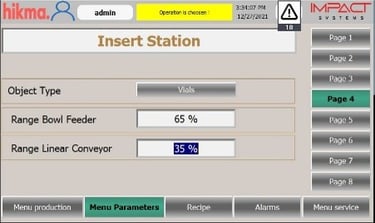
Automated Tablet / Capsule Lines Modernization
We specialize in refurbishing pharmaceutical solid product lines, extending equipment lifetimes, and improving efficiency. Our upgrades include modern control systems with full Data Integrity solutions to secure electronic records. All refurbishments are validated to meet FDA, GMP, and 21 CFR Part 11 compliance requirements.
Reference Project
Upgrade of Tablet / Capsule Production Lines
Refurbishment of two solid-dose production lines, including capsule filling (PAM), coating (Gansons), tablet pressing (Accura and KILIAN), and blistering (Hoonga). Systems validated per 21 CFR Part 11 & GMP.
Hikma Pharmaceuticals - 2022
Integration of advanced, validated HMI units aligned with Data Integrity requirements.
Implementation of secure electronic records and audit trails, ensuring full compliance with FDA 21 CFR Part 11 and GMP standards.
Comprehensive qualification and validation (IQ/OQ/PQ) to guarantee regulatory approval and operational reliability.






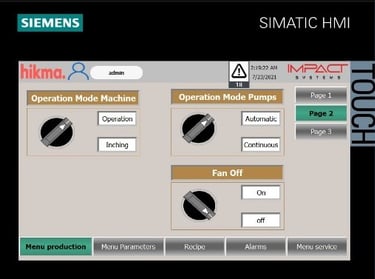
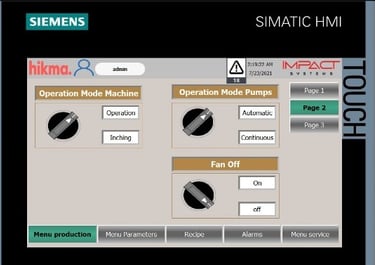
Machine Vision Systems
We provide high-speed, AI-enabled vision systems for real-time inspection and rejection of defective products. Using Cognex, Keyence, and Omron technologies, we detect issues like fill discrepancies, label misalignment, contamination, and product defects—ensuring flawless output without production delays. Promoted solutions could by complied with FDA guidelines, serialization, and trackability options when needed. Our solutions cover and are not limited to:
Solid & Semi-Solid Lines: Detects defects in tablets, capsules, and ointment tubes (shape, cracks, sealing).
Vial & Ampoule Lines: Inspects for particles, fill volume, sealing integrity, labeling, and surface issues.
Syrup Lines: Checks bottle quality, liquid levels, cap torque, and label accuracy.
Reference Project
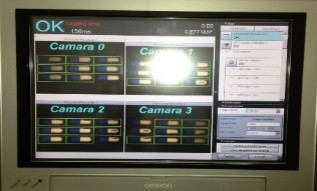
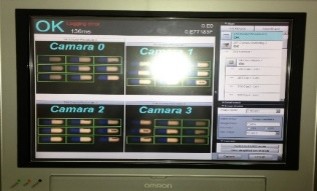
Machine vision solution for Syrup Production Line
Complete vision-based inspection and rejection solution (Omron platform), including electrical panel, smart vision camera, monitoring and reporting computer, and mechanical rejection system, delivered turnkey and fully validated.
DABUR Amla - 2022





Incubation Systems
Our systems deliver precise control of temperature, CO₂, humidity, and lighting for microbiological and stability testing in QC and R&D labs. Built on Siemens S7-1200 or Omron NX platforms, they support multi-chamber setups and Part 11-compliant logging.
Remote monitoring and deviation alerts
Audit-ready data reporting
Reference Project
Cooling Incubators Upgradation
Refurbishment and validation of two cooling incubators, Made by BINDER, ensuring full compliance with FDA 21 CFR Part 11 guidelines. ACDIMA - 2023


Modernization of Incubation Systems
Replacement and upgrading of obsolete components with modern, reliable systems.
Integration of advanced, validated control systems aligned with Data Integrity requirements.
Implementation of secure electronic records and audit trails, ensuring full compliance with FDA 21 CFR Part 11 and GMP standards.
Comprehensive qualification and validation (IQ/OQ/PQ) to guarantee regulatory approval and operational reliability.






Modern SCADA Systems
We deliver advanced SCADA solutions designed to optimize pharmaceutical manufacturing and monitoring processes. Our systems provide real-time data visualization, batch traceability, and seamless integration with MES and ERP platforms. Fully compliant with FDA, GMP, and 21 CFR Part 11, they ensure secure data integrity and regulatory readiness.
Reference Project
New Central SCADA systems
Implementation of two validated central SCADA systems (Siemens WinCC platform) for pharmaceutical production control and monitoring.
Hikma Pharmaceuticals - 2021


SCADA System Upgradation
Modernization of SCADA system (Schneider / Wonderware Intouch), including reprogramming, testing, and commissioning for process optimization for the Tomato Sauce production Line. Faragalla - 2021
Robotic Systems
We design and implement advanced robotic solutions tailored for pharmaceutical packaging lines, ensuring flexibility, precision, and regulatory compliance. Our systems handle a wide range of products, including blister packs, bottles, ampoules, vials, and tubes, while supporting secondary packaging operations such as case packing, labeling, and integration with serialization systems.
Key Features:
Vision-guided pick-and-place with high accuracy and adaptability.
Scalable modular design for future production expansion.
Reduced operational costs through optimized automation and minimized errors.
Enhanced traceability and electronic batch record support for full data integrity.
High-class branded robotic solutions such as Kuka, Kawasaki, Omron, and ABB


Automated Robotic Pharma Packaging


Automated Robotic Palletizing Systems
We deliver automated palletizing systems for cartons, crates, and shrink-wrapped products, ensuring stable, accurate stacking and maximum throughput. Integrated with WMS, our solutions streamline end-of-line operations and enhance efficiency with reliable 24/7 operation. Powered by KUKA, Fanuc, or Kawasaki robots, they combine high performance with ISO-compliant safety features.
Key Features:
Automated pallet handling with dispensers and stretch wrapping.
Intelligent grippers are adaptable to multiple product formats.
Seamless WMS/ERP integration for optimized logistics.
High-speed, accurate stacking with consistent load stability.
Full compliance with ISO safety standards.